In the near future, chemotherapy is expected to move from the ‘body flooding’ approach currently adopted, towards a more controlled and localized delivery.
Many of the current cancer chemotherapeutics work through mechanisms that do not differentiate between cancerous and healthy cells, resulting in the common adverse side effects associated with prolonged chemotherapy. For that reason, cancer therapy researchers are redirecting their efforts toward finding more sophisticated alternatives to administer chemotherapeutics instead of the classic ‘pill and syringe’ techniques.
The field of nanotechnology has received a fair share of attention over recent years due to the therapeutic potential it holds in terms of localized delivery of cancer drugs. Scientists have confidently shown that ultra-small particles or nanoparticles of various metals and synthetic material can be employed as vessels for cancer drugs.
The human body, however, is a hostile place for foreign substances.The synthetic nature of nanoparticles is not well received by the body’s immune system, nor is it compatible with the way that the body removes waste material, making nanoparticles toxic in their crude form.
Dr. Warren Chan from the Institute of Biomaterials and Biomedical Engineering at U of T; recent PhD graduate Dr. Vahid Raeesi; and Dr. Leo Chou from the Dana-Farber Cancer Institute have made a huge stride towards finding an effective nanotechnology-based approach for localized delivery of cancer drugs. Their technology does so while simultaneously overcoming the body’s immune rejection mechanisms and reducing toxicity.
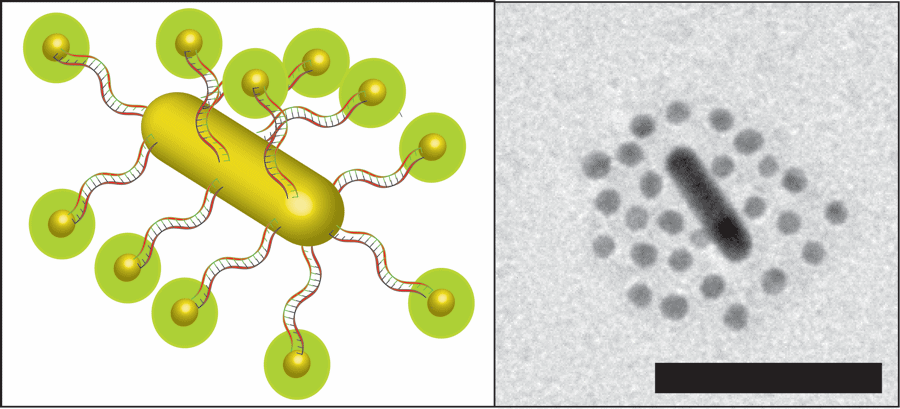
The novel design was the coming together of a number of previous findings from the Chan lab and other labs in the field. This cancer-targeted structure can be described as a ‘modular nanosystem,’ with ‘modular’ referring to its multi-components, and ‘nanosystem’ reflecting that is on the order of a nanometre or a millionth of a millimetre.
At the core of this intricate structure is a nanorod, an oblong structure made of gold, dubbed by Raeesi as a “nano heat generator” due to its ability to generate heat when struck by light of a certain energy. This core is orbited by a number of spherical gold nanoparticles or satellites docked onto the nanorod using threads of DNA, commonly used by biological engineers as an adhesive due to its great flexibility and potential for precise design, as Raeesi explained.
DNA exists in nature as two strands or thread-like structures bound together along their length. This gives DNA the ability to firmly sandwich certain chemicals between the strands. Subjecting it to high temperatures results in the immediate separation of the strands. For that reason, the research team cleverly proceeded to soak the DNA strands used in the nanostructure with common cancer drugs like Doxorubicin.
As soon as the nanostructure is delivered into the heart of the tumour through the blood supply, infrared light, which is able to harmlessly penetrate the human body, can be shone at the tumour. This results in heat radiating from the gold nanorod, which in turn splits the DNA strands and — like a Trojan horse — releases the cancer-killing molecule. The core-emitted heat also doubles as a controlled way to damage the nearby cancer cells to provide an additive effect, leaving distant healthy cells unharmed.
Raeesi emphasized the importance of the nanoparticles’ size, dictating it as necessary for the particles to be able to pass through the pore in the walls of the blood vessels feeding the tumours. Previous nanoparticle designs of larger sizes led them to get stuck in the vicinity of the tumour and eventually pushed back into the bloodstream, reducing the amount of the drug that makes it to the tumour.
Additionally, the modular, multi-unit nature of this novel nanosystem means that after finishing the job, the now disconnected parts can be easily removed from the body, a feature that is missing from earlier, single-unit designs.
A primary feature of the nanosystem is that the orbiting satellites are covered by a layer of a plastic-like substance or polymer known as polyethylene glycol. Although technically synthetic in nature, this substance was curiously found to interfere with white blood cells, the components responsible for the attack of foreign substances, helping the nanoparticles to escape the body’s immune system.
But why not put the protective polymers right on the nanorod? It all comes to ‘bioaccumulation,’ or as Raeesi describes it, the percentage of particles that make it into the tumour. Putting the polymers on the spheres results in higher degree of surface coverage that cannot be achieved by putting the polymer directly on the nanorod core. This in turn provides a better disguise against the body’s immunity and a higher chance of an uninterrupted journey to the tumour.
Raeesi hopes that his research along with others’ will pave the way to refining the system towards targeting metastasized and deeply embedded tumours, as well as developing systems with tumour-imaging properties.